
Newsroom
Innovative Drug-Delivery Patch Enhancing Heart Recovery Post-Attack

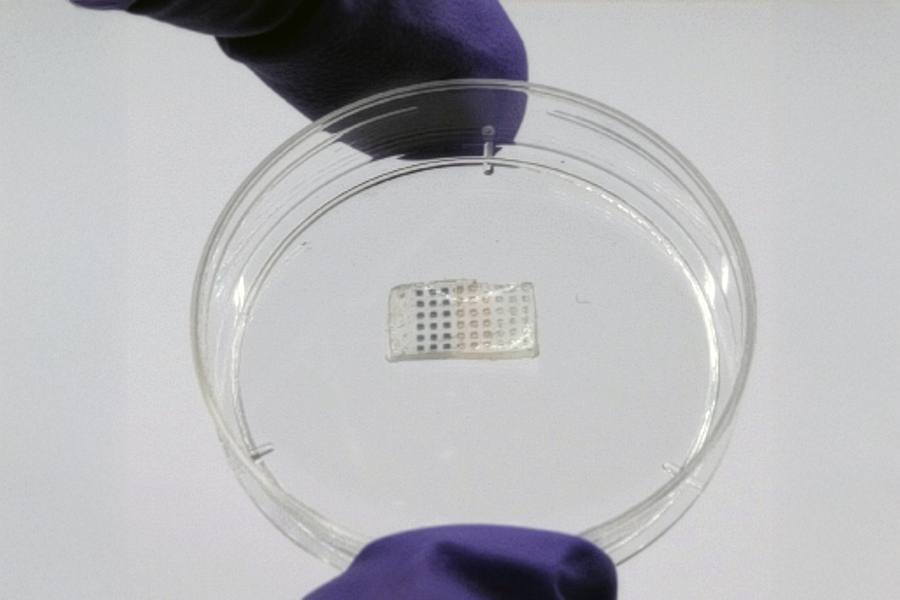
Engineers at MIT have innovatively developed a flexible drug-delivery patch specifically designed for application on the heart following a heart attack, aimed at enhancing healing and regeneration of cardiac tissue.
This advanced patch is engineered to carry multiple medications, which can be released at designated intervals according to a pre-set schedule. In animal studies, this approach demonstrated a remarkable 50% reduction in damaged heart tissue, alongside significant improvements in cardiac function.
If this patch receives approval for human use, it holds the potential to aid heart attack survivors in regaining a substantial portion of their cardiac function, surpassing current recovery capabilities.
“After a major heart attack, the damaged cardiac tissue often fails to regenerate effectively, leading to a lasting decline in heart function,” explains Ana Jaklenec, a principal investigator at MIT’s Koch Institute for Integrative Cancer Research. “Our mission is to restore this function, enabling individuals to reclaim a stronger, more resilient heart post-myocardial infarction.”
Jaklenec and Robert Langer, a distinguished professor at MIT and an influential member of the Koch Institute, co-authored the study published in Cell Biomaterials, with former MIT postdoc Erika Wang serving as the lead author.
The concept of programmed drug delivery is central to this innovation. After a heart attack, many patients require bypass surgery to enhance blood flow to the heart; however, this procedure does not repair the damaged cardiac tissue. The MIT team aimed to create a patch that could be applied concurrently with surgical intervention.
This novel patch is designed to deliver therapeutic agents over an extended period to promote tissue healing. Unlike conventional systems that release drugs all at once, this timed delivery method aligns treatment with the body’s recovery process.
“We aimed to explore the possibility of providing a precisely coordinated therapeutic intervention to facilitate heart healing right at the damage site during open-heart surgery,” Jaklenec remarks.
To achieve this goal, researchers adapted previously developed drug-delivery microparticles, resembling small capsules akin to coffee cups with lids. These capsules are constructed from a polymer known as PLGA and can be sealed with therapeutic agents.
By adjusting the molecular weight of the polymers used for the lids, the research team can regulate their degradation rates. This allows for programmed release of the encapsulated drugs at specific intervals post-implantation.
The team developed a regimen involving three drugs that promote heart healing through distinct mechanisms. The first particles release neuregulin-1, which is a growth factor that prevents cell death. At subsequent time points, particles release VEGF, another growth factor that encourages blood vessel formation around the heart. The final batch releases GW788388, a molecule that inhibits scar tissue formation post-heart attack.
“Tissue regeneration follows a precisely timed series of steps,” Jaklenec explains. “Dr. Wang created a system that delivers essential components at optimal times, mirroring the body’s natural healing sequence.”
The researchers integrated these particles into thin sheets of a robust yet flexible hydrogel, similar in feel to contact lenses. This biocompatible hydrogel is composed of alginate and PEGDA and gradually breaks down within the body. The study focused on compact patches measuring only a few millimeters.
“By encapsulating arrays of these particles in a hydrogel patch, we can surgically implant it into the heart, effectively programming the treatment into the material,” Wang states.
Upon creating these patches, the researchers tested them on heart tissue spheres containing cardiomyocytes derived from induced pluripotent stem cells, as well as endothelial cells and human ventricular cardiac fibroblasts—critical components for heart function.
Exposing these spheres to low-oxygen conditions that simulate a heart attack, they placed the patches atop them. Results indicated enhanced blood vessel growth, increased cell survival rates, and reduced fibrosis.
In rat models of heart attack, treatment with the patch yielded significant benefits. Compared to untreated controls or IV drug injections, rats receiving the patch experienced a 33% increase in survival rates and a 50% decrease in damaged tissue, along with marked improvements in cardiac output.
The patches are designed to dissolve gradually over time, forming a thin layer without interfering with the heart’s mechanical functions within a year.
“This presents a significant opportunity to merge drug delivery with biomaterials for innovative patient treatments,” Langer adds.
Of the medications investigated in this research, neuregulin-1 and VEGF have undergone clinical trials for heart conditions; however, GW788388 has primarily been tested in animal studies. The research team aims to further evaluate their patches in additional animal models, with aspirations for future clinical trials.
Currently, implantation of the patch requires surgical intervention; however, researchers are considering integrating these microparticles into stents capable of delivering drugs according to a programmed schedule.



