
Newsroom
Revolutionary Wireless Implants for Non-Invasive Brain Disease Treatment
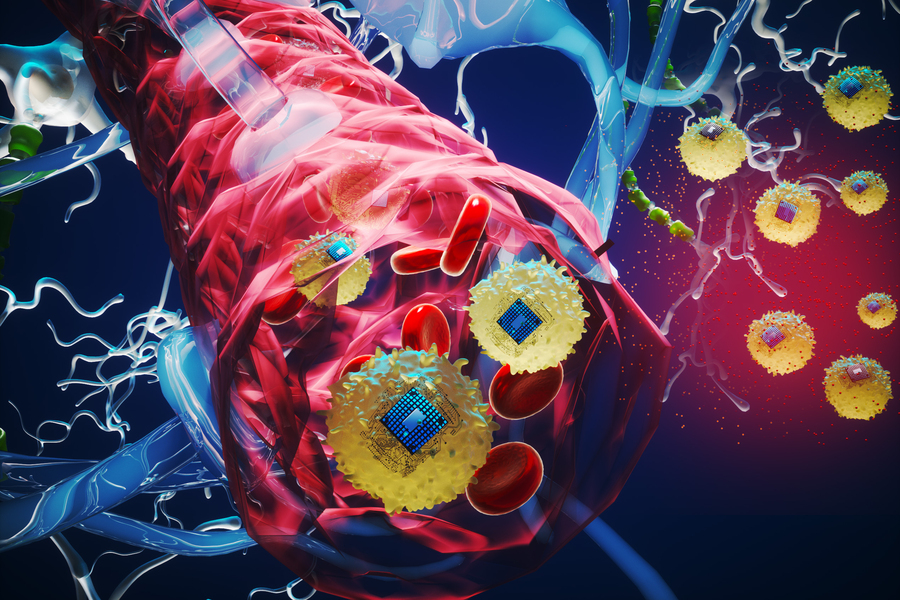
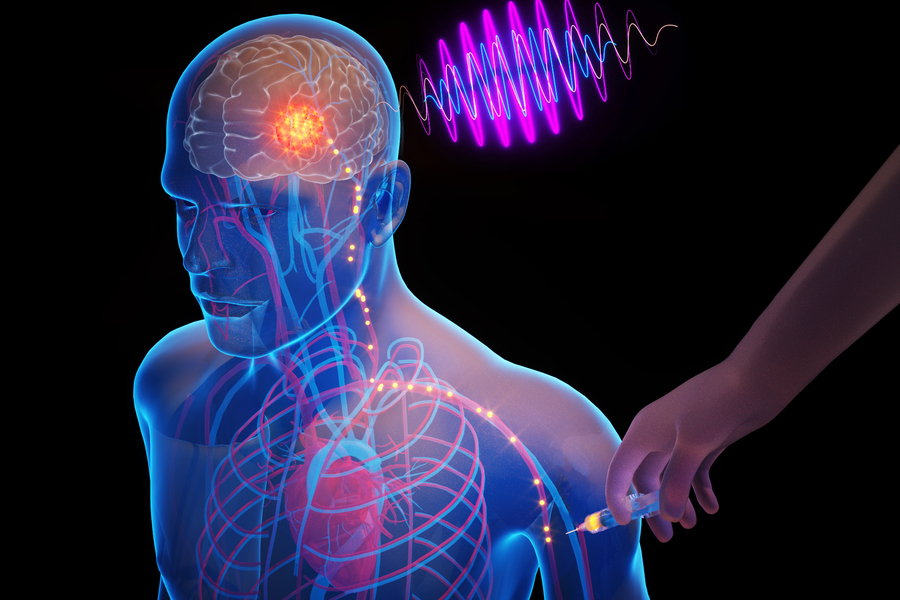
Imagine a future where clinicians can insert tiny electronic chips into the brain through a simple arm injection, allowing for precise electrical stimulation of targeted areas. This innovative approach could revolutionize the treatment of severe brain disorders, significantly reducing the risks and costs associated with traditional surgeries.
Researchers at MIT are making significant strides toward this vision by developing microscopic, wireless bioelectronics. These advanced devices are capable of navigating through the body’s circulatory system and autonomously implanting themselves in specific brain regions to deliver focused treatments.
In recent studies involving mice, these minuscule implants demonstrated the ability to locate and travel to designated brain areas without human intervention. Once they reach their targets, they can be wirelessly powered to provide neuromodulation—an electrical stimulation technique that has shown potential in treating conditions such as brain tumors, Alzheimer’s disease, and multiple sclerosis.
A remarkable feature of these implants is their biocompatibility. By integrating the electronic devices with living biological cells before injection, they evade the immune system’s defenses and successfully cross the blood-brain barrier while preserving its protective functions.
The research team has showcased the application of this technology, termed ‘circulatronics,’ to address brain inflammation—a critical contributor to various neurological disorders. The implants achieve localized neuromodulation with impressive precision, targeting areas within mere microns.
Unlike conventional brain implants that incur exorbitant costs and require risky surgical procedures, circulatronics holds promise for making therapeutic brain interventions accessible to a wider population by eliminating surgical needs.
The development of circulatronics has spanned over six years. Each device, measuring about one billionth of a grain of rice, consists of organic semiconducting polymer layers encased in metallic layers, forming an electronic heterostructure. These devices are crafted using CMOS-compatible processes at MIT.nano facilities and subsequently integrated with living cells to form hybrid cell-electronics systems.
The key to the operation of these devices lies in their high wireless power conversion efficiency, allowing them to function effectively deep within the brain while still generating sufficient energy for neuromodulation.
Researchers utilize a chemical reaction to bond these electronic devices to cells. In their latest study, they fused the electronics with monocytes—immune cells that naturally target inflammation—while applying a fluorescent dye for tracking purposes as the devices traversed the intact blood-brain barrier and self-implanted at their designated sites.
While this study focused on brain inflammation, the team aims to explore other cell types and engineer them to target specific brain regions more effectively.
The unique fusion of electronics with living cells enhances the versatility of the devices. This integration not only helps them evade immune attacks but also facilitates seamless travel through the bloodstream and across the blood-brain barrier without invasive procedures.
Through rigorous experimentation over four years, the team perfected a technique for noninvasive crossing of the blood-brain barrier by combining electronics with living cells.
Additionally, the diminutive size of circulatronics devices provides a level of precision unattainable by traditional electrodes. Their ability to self-implant results in millions of stimulation sites that conform precisely to target regions.
The biocompatible nature of these tiny devices allows them to coexist with neurons without adverse effects. Comprehensive biocompatibility tests confirmed that circulatronics can safely integrate among neurons without disrupting cognitive or motor functions.
Once implanted, clinicians or researchers can use external transmitters to emit electromagnetic waves in the form of near-infrared light, powering the devices and facilitating electrical stimulation of neurons.
Currently, the Sarkar lab is advancing their technology to tackle various diseases including brain cancer, Alzheimer’s disease, and chronic pain. The unique characteristics and self-implantation capabilities of circulatronics make them particularly promising for treating multifocal brain tumors like glioblastoma, which may be too small for conventional imaging techniques to detect.
This platform technology has broad implications for treating numerous neurological conditions and mental health disorders. Furthermore, its applications could extend beyond the brain to other regions of the body in future developments.
The research team aims to initiate clinical trials within three years through their newly established startup, Cahira Technologies. They are also investigating the incorporation of additional nanoelectronic circuits into their devices for enhanced functionalities such as sensing and data analysis capabilities.
In conclusion, these innovative electronic devices create a novel synergy between electronics and biological systems, paving the way for transformative treatments of neural diseases where existing therapies have fallen short. The researchers envision a future where human limitations are transcended through this groundbreaking technology.



