
Newsroom
Innovative CO2 Conversion Technology for Sustainable Chemical Production
Innovative chemical engineers have developed a groundbreaking method to convert carbon dioxide into carbon monoxide, a vital precursor for producing valuable compounds such as ethanol and various fuels. This process, if scaled for industrial applications, holds the potential to significantly reduce carbon dioxide emissions from power plants and other sources, contributing to a decrease in greenhouse gases released into the atmosphere.
Revolutionizing CO2 Conversion with DNA Tethering
“This approach enables the conversion of carbon dioxide from emissions or from oceanic absorption into economically valuable chemicals. It represents a significant step towards decarbonization, transforming CO2, a greenhouse gas, into useful materials for chemical manufacturing,” explains an assistant professor specializing in chemical engineering and the lead researcher of this study.
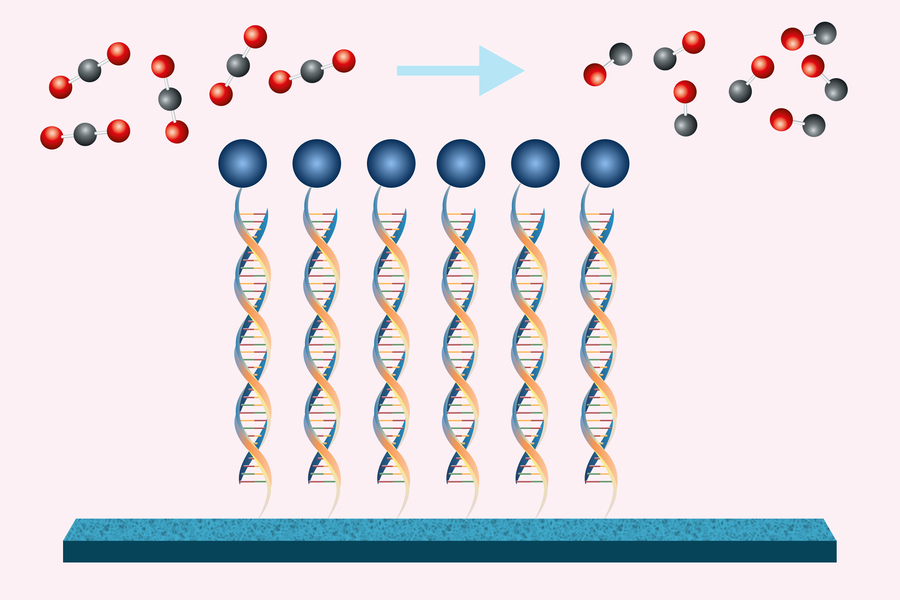
The innovative technique employs electricity to facilitate the chemical transformation, aided by a catalyst that is anchored to the electrode surface via DNA strands. This DNA structure acts like Velcro, keeping all reaction components in close proximity and enhancing reaction efficiency compared to traditional methods where components are dispersed in solution.
To advance this technology, a new company has been established, focusing on further development. The leading author of the research paper published in the Journal of the American Chemical Society Au has emphasized the importance of this work in the ongoing quest to utilize CO2 effectively.
Catalysts and Electrochemical Innovation
The initial step in converting carbon dioxide into useful products involves transforming it into carbon monoxide. Traditional methods using electricity can be prohibitively expensive due to high energy requirements. Researchers have explored the use of electrocatalysts to accelerate reactions and lower energy input.
Among the catalysts used are porphyrins, metal-containing molecules similar in structure to heme, which is responsible for oxygen transport in blood. In this electrochemical reaction, carbon dioxide is dissolved in water within an electrochemical device that houses an electrode driving the reaction. However, efficiency has been limited as the interaction between carbon dioxide and catalysts at the electrode surface occurs infrequently.
To enhance reaction frequency and boost electrochemical conversion efficiency, researchers have worked on attaching catalysts to the electrode surface using DNA. This material is not only cost-effective but also chemically modifiable, allowing precise control over interactions between different strands.
DNA as a Molecular Velcro
The team employed two “chemical handles”—one on the DNA and one on the electrode—to create a permanent bond. A complementary DNA sequence was then linked to the porphyrin catalyst. This setup allows the catalyst to reversibly bind to the DNA on the electrode when added to the solution, akin to Velcro.
Once established, researchers apply a potential to the electrode, enabling the catalyst to convert dissolved carbon dioxide into carbon monoxide while simultaneously generating hydrogen gas from water. When catalysts degrade, they can be easily replaced by heating the system to break reversible bonds between DNA strands.
This innovative method has achieved a remarkable Faradaic efficiency of 100%, indicating that all electrical energy input directly contributes to chemical reactions without any wastage.
In contrast, traditional systems without DNA tethering only achieve about 40% efficiency.
Scalability and Future Directions
The technology’s scalability for industrial use is promising due to the low cost of carbon electrodes compared to conventional metal electrodes. Additionally, the catalysts employed are cost-effective as they do not rely on precious metals and only require minimal concentrations on electrode surfaces.
Future plans include experimenting with different catalysts to produce additional products such as methanol and ethanol using this method. The newly established company is dedicated to developing this technology for potential commercial applications.
This research initiative received funding from various organizations including the U.S. Army Research Office, CIFAR Azrieli Global Scholars Program, MIT Energy Initiative, and MIT Deshpande Center.



