
Newsroom
Innovative Zinc-Air Battery for Autonomous Micro Robots in Medical and Industrial Applications
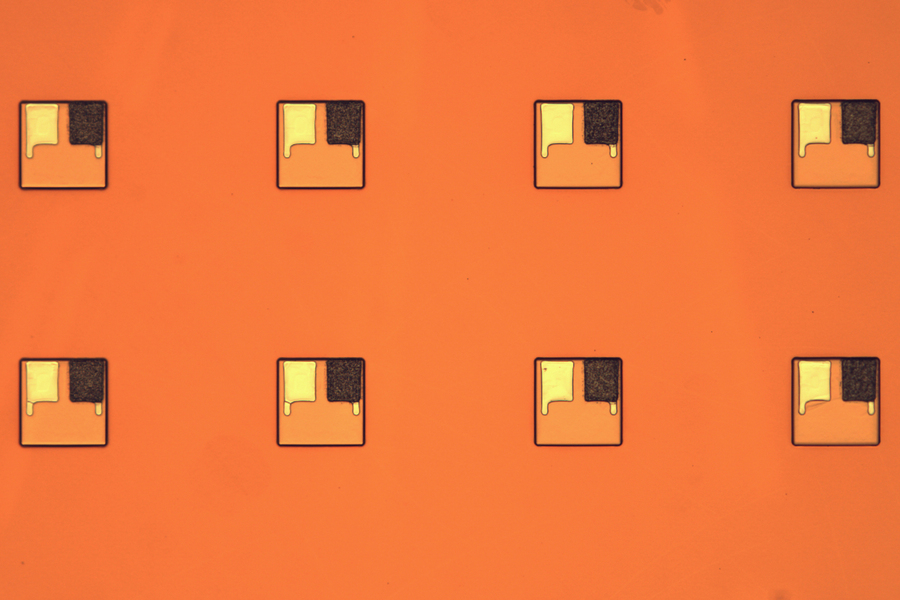
Engineered by researchers at MIT, a groundbreaking tiny battery has been developed, potentially revolutionizing the deployment of cell-sized autonomous robots for drug delivery within the human body and other applications, such as detecting leaks in gas pipelines.
A Revolutionary Power Source for Microrobots
Measuring just 0.1 millimeters in length and 0.002 millimeters in thickness—approximately the thickness of a human hair—this innovative battery captures oxygen from the surrounding air to oxidize zinc, generating a current with a voltage of up to 1 volt. This power output is sufficient to operate small circuits, sensors, or actuators.
“We believe this technology will significantly advance the field of robotics,” stated Michael Strano, a Chemical Engineering professor at MIT and the lead author of the study published in Science Robotics. “We are integrating robotic functionalities directly onto the battery and assembling these components into fully functional devices.”
Challenges in Powering Miniature Robots
Strano’s lab has dedicated years to the development of miniature robots capable of sensing and responding to environmental stimuli. A key hurdle has been ensuring these tiny robots have an adequate power supply.
While previous research has demonstrated that microscale devices can be powered using solar energy, this method requires a constant light source, effectively tethering the robots to their power supply. These systems, often referred to as ‘marionettes,’ lack autonomy. A battery-powered design, however, would enable robots to operate independently and explore areas previously deemed inaccessible.
“Marionette systems don’t require batteries since they draw energy externally,” Strano explained. “For a small robot to navigate freely, it must possess a higher degree of autonomy, making an internal battery essential.”
Innovative Zinc-Air Battery Design
To enhance the autonomy of these robots, Strano’s team opted for a zinc-air battery, known for its longevity and high energy density, frequently used in hearing aids.
The designed battery consists of a zinc electrode linked to a platinum electrode, encased within a polymer strip known as SU-8, commonly utilized in microelectronics. Interaction with atmospheric oxygen causes zinc oxidation, releasing electrons that flow to the platinum electrode and generating current.
The researchers demonstrated that this compact battery could effectively power an actuator, illustrated by a robotic arm capable of raising and lowering. Additionally, it could energize a memristor—an electrical component capable of retaining information by altering its resistance—as well as a clock circuit, enabling robotic devices to track time.
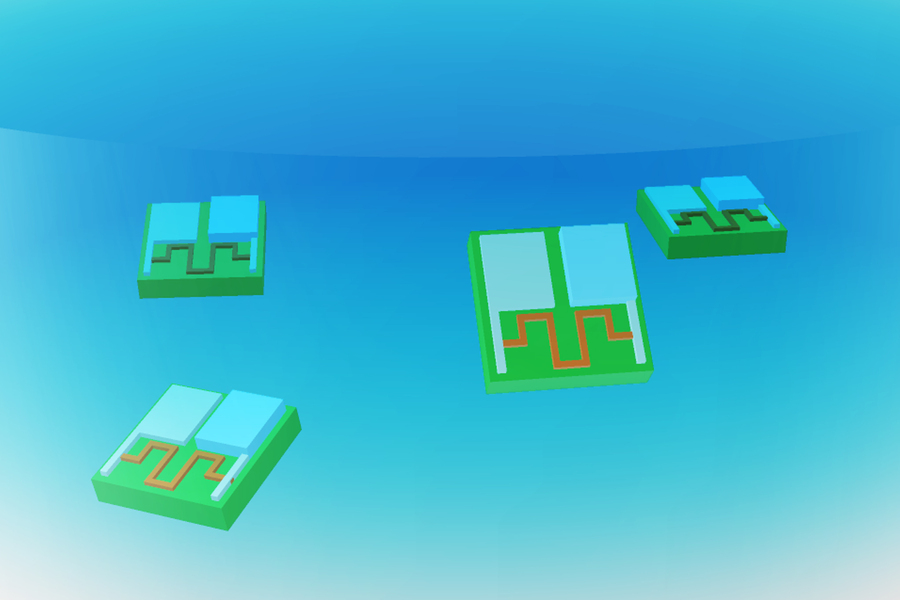
Applications and Future Directions
Moreover, the battery supplies sufficient energy to operate two types of sensors that alter their electrical resistance when exposed to specific chemicals. One sensor is crafted from atomically thin molybdenum disulfide, while the other utilizes carbon nanotubes.
“We are establishing foundational building blocks to develop functions at the cellular level,” Strano emphasized.
Although the current study utilized a wire to connect the battery to external devices, future endeavors will focus on integrating the battery directly into robotic structures.
“This innovation will serve as the cornerstone for many of our robotic initiatives,” Strano remarked. “Just as an electric car is built around its battery, we can construct robots centered on this energy source.”
One promising application involves designing miniature robots that could be injected into the human body to locate target areas and release medications like insulin. For medical use, these devices are envisioned to be constructed from biocompatible materials that disintegrate once their function is fulfilled.
Additionally, researchers are working on enhancing the voltage of this battery, which could unlock even more applications.
This research received funding from the U.S. Army Research Office, the U.S. Department of Energy, the National Science Foundation, and a MathWorks Engineering Fellowship.



